As we reported here the FDA yesterday changed the advice regarding the Covid vaccinations. The FDA simplified COVID vaccine schedules, and withdrew authorization for older COVID-19 vaccines targeting the virus’ original strain. Now, not only has the monovalent version been discontinued, but the dosage amount has changed.
It’s part of an overall move to simplify the COVID-19 vaccination process, the agency said in a statement. United States regulators are shifting towards a flu shot-like model for COVID-19 vaccines, where people get a single shot every year that’s updated annually to match the virus strain predicted to be in circulation.
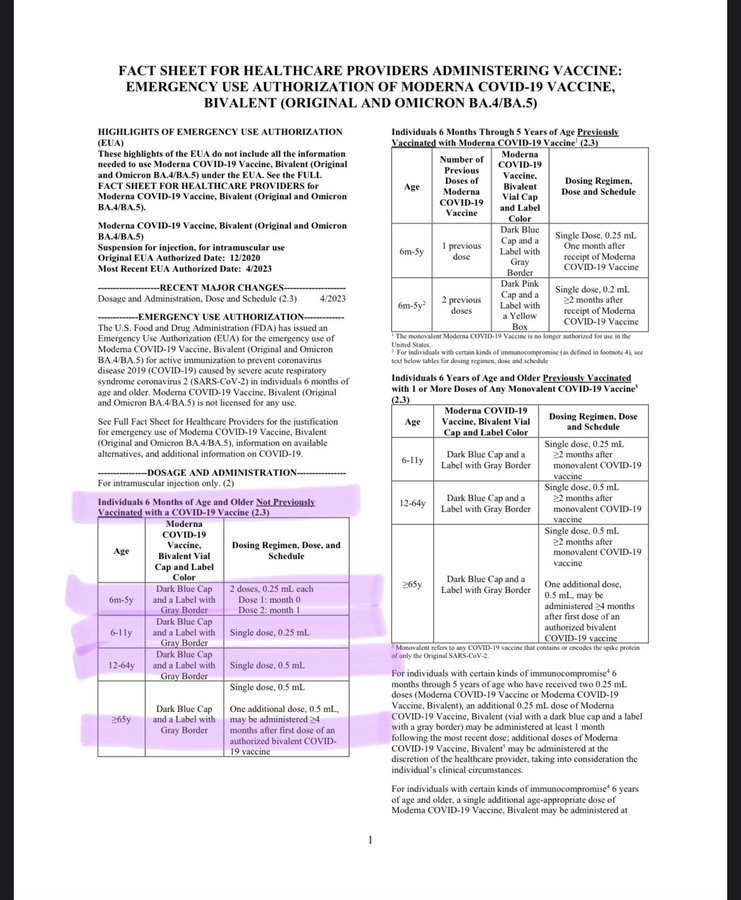
As this commentator observes, this month a child would have been dosed with 75% less than last month. Now, children 6 months through 5 years of age can either get two doses of the Moderna bivalent vaccine or three doses of the Pfizer-BioNTech vaccine. Questions are being raised regarding the data behind the decision.
Yesterday the @US_FDA revoked the authorization for all previously licensed Covid vaccines and then cut the dosage by 75% for all new vaccinations using the EUA bivalent vaccine. If you had taken your 6 year old to get vaccinated against Covid for the first time last month they would have given him a .50 ml shot then made you bring him back 4 weeks later for a second .50 ml shot in order for him to be considered fully vaccinated after 2 weeks. A total dosage of 100 μg in just 4 weeks. If you took him in today he’d receive a single .25 ml (25 μg) shot and in 2 weeks time he’d be considered fully vaccinated. Where is the data informing this decision?
Yesterday the @US_FDA revoked the authorization for all previously licensed Covid vaccines and then cut the dosage by 75% for all new vaccinations using the EUA bivalent vaccine.
— Mel (@Villgecrazylady) April 19, 2023
If you had taken your 6 year old to get vaccinated against Covid for the first time last month they… pic.twitter.com/g6PRAKGuoA